Janssen and Samsung Bioepis Settle STELARA (Ustekinumab) Litigation
LexBlog IP
DECEMBER 13, 2023
The post Janssen and Samsung Bioepis Settle STELARA (Ustekinumab) Litigation appeared first on Big Molecule Watch.

LexBlog IP
DECEMBER 13, 2023
The post Janssen and Samsung Bioepis Settle STELARA (Ustekinumab) Litigation appeared first on Big Molecule Watch.

LexBlog IP
MARCH 6, 2024
announced it has signed a settlement agreement with Bayer Inc. The settlement resolves multiple patent infringement proceedings in the Federal Court of Canada. Under the agreement, Biocon can launch YESAFILI in Canada no later than July 1, 2025. On March 4, Biocon Biologics Ltd. and Regeneron Pharmaceuticals, Inc.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Biswajit Sarkar Copyright Blog
DECEMBER 19, 2023
As India aims for a $5 trillion economy, the MSME ministry sets ambitious goals, aiming to elevate their GDP contribution by up to 50% by 2025. Subsidies offered for Patent and Trademark (in selected categories) registration, encouraging innovation and protecting intellectual property.

LexBlog IP
NOVEMBER 5, 2023
As we covered previously , Janssen and Amgen settled their BPCIA litigation regarding Amgen’s ustekinumab biosimilar product in May 2023. ” (CMS’s position on this has been challenged in pending litigation.) WEZLANA is the first product to be approved as a biosimilar to STELARA.

LexBlog IP
JANUARY 25, 2024
Janssen and Amgen settled their BPCIA litigation regarding Amgen’s ustekinumab biosimilar product in May 2023. ” (CMS’s position on this has been challenged in pending litigation.) WEZLANA is the first product to be approved as a biosimilar to STELARA.
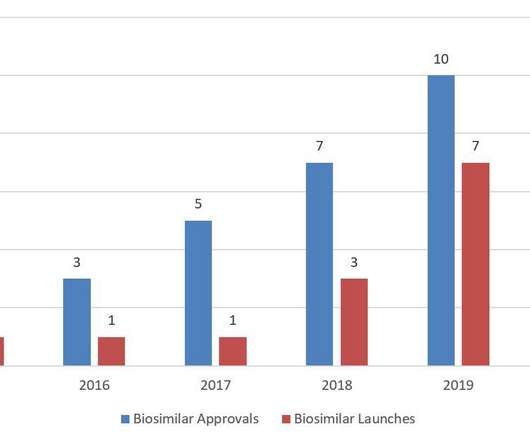
Fish & Richardson Trademark & Copyright Thoughts
FEBRUARY 4, 2021
Yet 2020 saw a slowdown in biosimilar activity with the lowest number of annual biosimilar approvals since 2016 and fewer product launches than 2019—as well as a decrease in district court litigation and post-grant proceedings. BPCIA Litigation. Antitrust Litigation. No earlier than July 31, 2023 per settlement.

Patently-O
MAY 15, 2024
This wave of RFCs includes significant proposals aimed at adjusting patent fees for fiscal year 2025, refining terminal disclaimer practices, and addressing the impact of artificial intelligence on prior art and patentability. 325(d) considerations, parallel and serial petitions, and settlement-related terminations.
Let's personalize your content