NPE Showcase – Sockeye Licensing
LexBlog IP
FEBRUARY 7, 2023
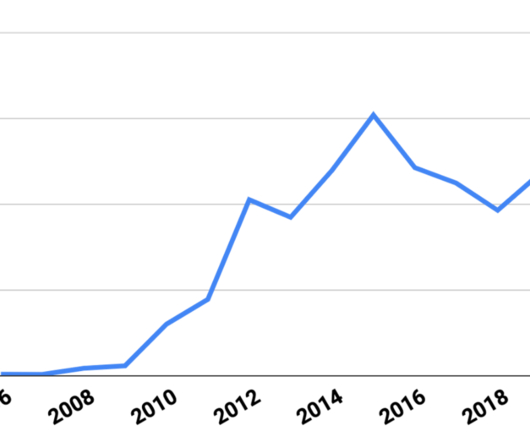
Sockeye has sued approximately 80 defendants since it began its patent infringement campaigns in 2015. For example, Sockeye sued a group of electronics companies in 2015 and sued the same defendants again in 2022 with at least some of the same patents. So what happened?








Let's personalize your content